Clinical Outcomes After NTI Generic Switches: What Studies Show
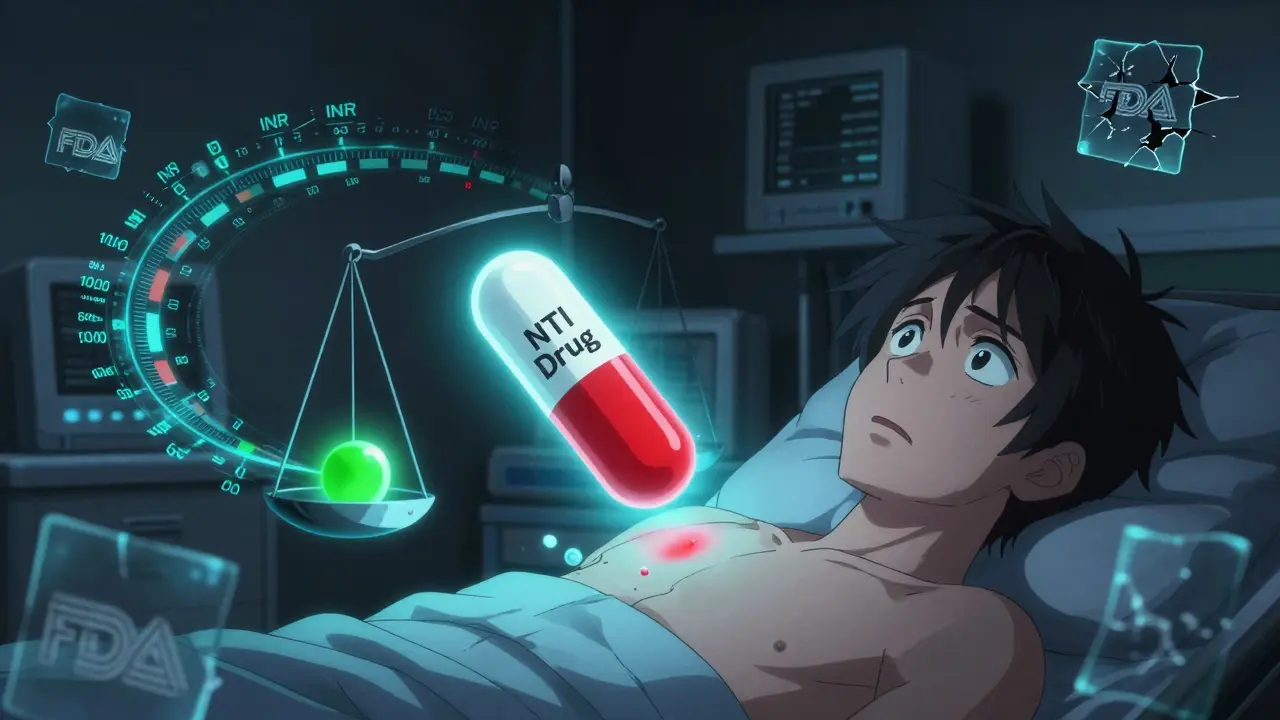
When a doctor prescribes a medication like warfarin, phenytoin, or cyclosporine, they’re not just picking a drug-they’re picking a precise dose that keeps a patient alive. These are NTI drugs, or narrow therapeutic index drugs. A tiny change in blood levels-just 10%-can mean the difference between effective treatment and a life-threatening reaction. That’s why switching from a brand-name version to a generic isn’t as simple as swapping one pill for another.
What Makes a Drug an NTI Drug?
NTI drugs have a razor-thin safety margin. The amount needed to work is almost the same as the amount that can hurt you. The FDA defines them as drugs where small changes in blood concentration can cause serious harm: organ failure, uncontrolled seizures, blood clots, or transplant rejection. Examples include warfarin (a blood thinner), phenytoin and levetiracetam (anti-seizure meds), levothyroxine (thyroid hormone), digoxin (heart medication), and cyclosporine and tacrolimus (immunosuppressants).
Most drugs have a wide window-take a little more or less, and you’re fine. Not NTI drugs. For warfarin, a 10% drop in blood levels might mean a clot forms. A 10% rise could cause internal bleeding. For cyclosporine, even a 5% increase after a switch can trigger kidney damage in transplant patients. That’s why these drugs require constant monitoring and why switching formulations isn’t taken lightly.
How Are Generics Tested? The Bioequivalence Gap
When a generic drug is approved, the FDA requires it to be bioequivalent to the brand-name version. That means the generic must deliver the same amount of active ingredient into the bloodstream, within a range of 80% to 125% of the brand. Sounds fair, right? But here’s the problem: for NTI drugs, that 45% swing is enormous.
Imagine two generic versions of phenytoin. One delivers 80% of the brand’s concentration. Another delivers 125%. If a patient switches from one generic to another, their blood level could jump by more than 50%. That’s not a minor fluctuation-it’s a clinical emergency waiting to happen.
Other countries know this. Canada and the European Medicines Agency require NTI generics to stay within 90% to 111% of the brand. That’s a much tighter window. The FDA still uses the broader range for all drugs, including NTIs, despite warnings from experts for decades. In 2022, the FDA began drafting product-specific guidelines for NTI drugs, signaling a possible shift. But as of early 2026, the old rules still apply nationwide.
What Happens When Patients Switch?
Real-world data tells a messy story. For some NTI drugs, switching works fine. For others, it’s a gamble.
Warfarin: Some studies show no difference in bleeding or clotting events between brand and generic warfarin in controlled trials. But in real clinics? A 2021 analysis of 36,911 patients found that 42% of those switched to generic warfarin needed dose adjustments within weeks. Their INR levels-measuring blood thinning-became unstable. Pharmacists reported higher rates of follow-up visits and emergency lab draws after the switch.
Antiepileptic drugs: This is where things get scary. A review of 760 epilepsy patients found that switching to generic levetiracetam led to increased seizures in a significant number. Patients reported blurred vision, memory loss, mood swings, and aggression-symptoms that vanished when they switched back to the brand. One study documented 50 patients who had breakthrough seizures after a generic switch; nearly half had lower drug levels at the time. Neurologists in 73% of U.S. states now have legal protections to prevent automatic substitution of antiepileptic generics without the prescriber’s consent.
Immunosuppressants: For transplant patients, a failed switch can mean losing the new organ. One study of 73 patients switching from Neoral (brand cyclosporine) to a generic found that 17.8% needed immediate dose adjustments. Trough levels jumped from 234 ng/mL to 289 ng/mL in just two weeks. That’s a 23% increase-enough to cause kidney toxicity. On the flip side, some studies show tacrolimus generics are stable when tested under tighter NTI standards. But even then, variability between manufacturers is real. One lab found active ingredient levels ranging from 86% to 120% across different generic brands of tacrolimus.
Who’s Watching? The Role of Pharmacists and Patients
Pharmacists are on the front lines. A national survey of 710 pharmacists showed that 82% still substitute generic NTI drugs routinely. But 41% recommend extra monitoring after the switch. Among those who work outside big chain pharmacies, skepticism is higher. Female pharmacists were more likely to voice concerns. Many say they’ve seen patients come back with complaints after a switch-sometimes too late.
Patients aren’t silent. On forums like Reddit’s r/transplant, people share stories of rejection episodes after switching from Neoral to generic cyclosporine. On epilepsy support boards, threads are filled with posts like: “Switched to generic levetiracetam. Had three seizures in a week. Switched back. Zero since.” The PatientsLikeMe platform shows that while 58% of warfarin users report no issues, 42% say their INR became unpredictable after the switch.
These aren’t outliers. They’re signals. The system assumes bioequivalence equals therapeutic equivalence. But for NTI drugs, that’s not always true.
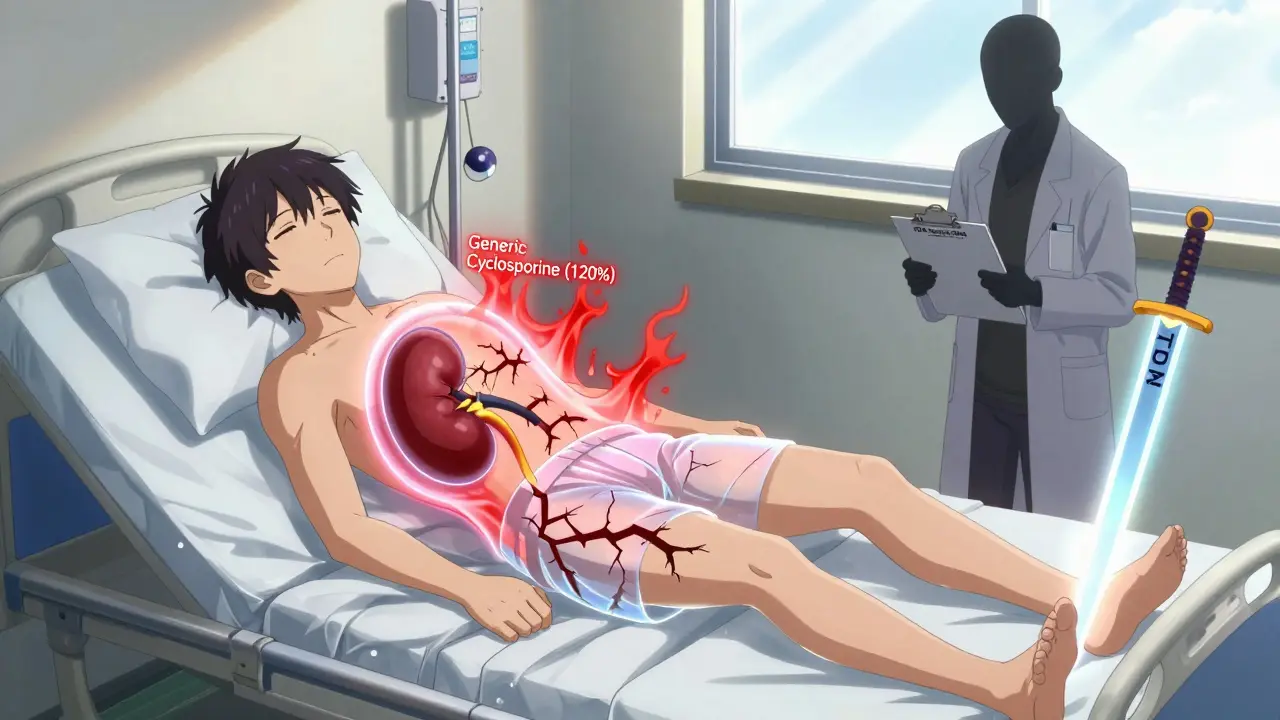
What Should You Do If You’re on an NTI Drug?
If you take one of these medications, here’s what you need to know:
- Ask your doctor if your drug is an NTI drug. If you’re on warfarin, phenytoin, cyclosporine, or levothyroxine, you are.
- Ask if you can stay on the same brand or generic version. If you’ve been stable for months or years, switching offers no benefit-only risk.
- Know your pharmacy’s substitution policy. Some states allow automatic substitution unless the doctor writes “dispense as written.” Request that if you’re on an NTI drug.
- Monitor closely after any switch. For warfarin, get an INR test within 3-7 days. For cyclosporine or tacrolimus, ask for a trough level check at 2 and 4 weeks. For antiepileptics, track seizure frequency and mood changes.
- Report any changes to your doctor immediately-even if they seem minor. A headache or mood swing could be a sign your drug level shifted.
The Future of NTI Drug Substitution
Change is coming, but slowly. The FDA’s 2022 draft guidance is a step toward customizing bioequivalence standards for each NTI drug, instead of using a one-size-fits-all rule. Post-marketing surveillance is also tightening. In 2021, NTI drugs made up just 5% of generic prescriptions but 18% of all adverse event reports tied to generics.
Therapeutic drug monitoring (TDM)-regular blood tests to check drug levels-is becoming more common. Industry analysts predict a 15-20% rise in TDM tests over the next five years, mostly because of generic substitution concerns. Some researchers are exploring pharmacogenomics: testing a patient’s DNA to predict how they metabolize drugs like warfarin or phenytoin. That could one day make dosing more personal and less dependent on formulation.
For now, the message is clear: not all generics are equal. And when it comes to NTI drugs, the stakes are too high to assume they are.
Are all generic drugs unsafe for NTI medications?
No, not all generics are unsafe. Many patients switch successfully without issues, especially with drugs like warfarin and digoxin. But the risk is higher than with other medications. The problem isn’t the generic label-it’s the variability between different generic manufacturers and the lack of tighter testing standards. Some generics perform consistently; others don’t. That’s why staying on the same version, and monitoring closely after any switch, is critical.
Can my pharmacist switch my NTI drug without telling me?
In most states, yes-unless your doctor writes "dispense as written" or "no substitution" on the prescription. Pharmacy laws vary by state. Some states require pharmacists to notify patients or doctors before switching NTI drugs. Others don’t. Always ask your pharmacist if a substitution was made. If you’re on an NTI drug, it’s your right to know.
Why does the FDA allow 80-125% bioequivalence for NTI drugs?
The FDA uses the same standard for all drugs because it’s simpler to enforce. Changing the rule would require new testing protocols, more data, and more time to approve generics-raising costs and slowing access. But experts argue that for NTI drugs, the current standard is outdated. The FDA acknowledges the concern and is working on product-specific guidelines, but no nationwide change has been implemented yet.
Is levothyroxine an NTI drug?
Yes. Levothyroxine is one of the most common NTI drugs. Small changes in blood levels can cause symptoms like fatigue, weight gain, heart palpitations, or bone loss. The FDA has issued multiple warnings about switching brands or generics of levothyroxine. Many endocrinologists recommend staying on the same formulation and checking TSH levels 6-8 weeks after any switch.
What should I do if I think my generic NTI drug isn’t working?
Don’t stop taking it. Contact your doctor right away. Keep a log of symptoms-seizures, bruising, heart rate changes, mood shifts, or fatigue. Ask for a blood test to check drug levels if applicable. Request to return to your previous brand or generic. If your doctor agrees, have them write "dispense as written" on future prescriptions. Your safety depends on consistency.


So let me get this straight-we let pharmacies swap life-or-death meds like they're trading baseball cards?
Yeah that sounds like a brilliant plan.
This is why American generics are the best. If you can't handle a little variability you shouldn't be on meds at all. We lead the world in innovation and you're crying over 45% swings? Get over it.
Canada's 90-111% rule isn't just smart-it's basic math. We don't gamble with transplant patients here. Funny how the US calls itself the leader in medicine while letting pharmacists play Russian roulette with cyclosporine.
I work in a clinic in Delhi and we see this all the time. Patients come in with seizures after switching generics because the pharmacist didn't even know what NTI meant. We have to manually check every single prescription for warfarin and levothyroxine because the system is broken. The FDA is stuck in 2005 and the pharmaceutical companies are laughing all the way to the bank. I've seen kids lose school years because their generic phenytoin was 120% potent. It's not just about money-it's about people. And honestly? If you're okay with this, you haven't met the people who've been burned by it. We need to fix this before someone dies because a pharmacist thought "bioequivalent" meant "same thing".
Studies show 58% of people are fine. Problem solved.
I'm Canadian and I gotta say-our system isn't perfect but at least we don't treat human lives like batch numbers on a soda can. I had a cousin who got a kidney transplant and they switched her cyclosporine generic without telling her. She spent three weeks in the hospital. They finally admitted the generic had 18% higher absorption. We don't do that here anymore. Shame the US hasn't caught up.
I'm a pharmacist in Ohio and I see this daily. I hate swapping NTI generics. I've had patients cry because they had a seizure after switching. I always call the doctor to confirm. Most docs don't even know the rules. But corporate says "substitute unless told otherwise" so we do. It's not about what's right-it's about what the system allows. I just wish more people knew how dangerous this really is.
The bioequivalence range of 80-125% is statistically valid for most pharmaceuticals. However, the assumption of therapeutic equivalence for NTI drugs represents a significant clinical oversight. The pharmacokinetic variability, while within regulatory thresholds, exceeds the therapeutic window for drugs such as warfarin and cyclosporine. This necessitates a reevaluation of current FDA guidance in light of pharmacodynamic data and real-world adverse event reporting.
This is why we need to stop treating medicine like a commodity 🤕
People aren't widgets. Your thyroid isn't a lightbulb you can swap out for a cheaper version.
When your body's balance is this fragile, consistency isn't luxury-it's survival. 🙏
Wow so the FDA is bad and we should all just pay 10x more for brand names? What a shocker. Next you'll say we should ban generics entirely.
I'm a doctor in NYC and I've seen patients die because of this. The FDA is run by lobbyists. They don't care if you bleed out or reject a kidney. They care about how fast they can approve a generic so Big Pharma can make more money. This isn't a debate. It's a crime.
My mom's on levothyroxine and we switched generics last year. She got super tired, gained 15 lbs, and her heart was racing. We switched back and she's fine. I didn't even know this was a thing until it happened. Now I always check the label and ask the pharmacist. You'd think this would be common knowledge but it's not.
If you're too weak to handle a 10% change in your meds, maybe you shouldn't be alive. Stop being a hypochondriac and take your pills like an adult. This is why America's falling apart.