Why Healthcare Providers Weigh Risks vs Benefits of Medications
Medication Benefit-Risk Calculator
How to Use This Tool
Enter your condition and treatment options to see real-world benefit vs risk comparisons. This is for educational purposes only - always consult your doctor.
Your Benefit-Risk Assessment
Treatment Comparison
Every time a doctor prescribes a pill, they’re not just handing out a solution-they’re making a high-stakes gamble. The question isn’t just, Does this work? It’s, Is the cost worth it? For every medication, there’s a balance between what it can do for you and what it might do to you. That’s the core of benefit-risk assessment, and it’s the reason your doctor spends 15 to 20 minutes explaining side effects before you even leave the office.
It’s Not About Avoiding Side Effects-It’s About Managing Trade-Offs
No medication is risk-free. Even aspirin can cause internal bleeding. Antibiotics can wreck your gut. Blood pressure drugs can make you dizzy. But if you have a heart condition, that dizziness might be a small price to pay for avoiding a stroke. That’s the reality doctors face every day. They don’t pick drugs because they’re perfect. They pick them because they’re the least bad option for your specific situation. The U.S. Food and Drug Administration (FDA) requires every new drug to prove its benefits outweigh its risks before it hits the market. But that’s just the starting point. In the clinic, it gets personal. A drug that’s safe for a healthy 50-year-old might be dangerous for a 78-year-old with kidney disease. A treatment that saves lives in advanced cancer might be too harsh for someone with early-stage disease and years ahead of them.How Doctors Actually Do the Math
There’s no calculator for this. But there’s a framework. Doctors look at four things:- How serious is your condition? A life-threatening illness like metastatic melanoma justifies bigger risks. A mild headache? Not so much.
- What are the alternatives? If there’s a safer drug that works almost as well, that’s the one. But if all other options have failed, you’re willing to take a bigger chance.
- How strong is the benefit? Does the drug reduce your chance of death by 10%? Or 50%? A 40% improvement in survival for a terminal illness changes everything.
- How bad are the side effects? A 15% chance of nausea? Manageable. A 5% chance of liver failure? That’s a hard stop unless there’s no other choice.
Patients and Doctors Don’t Always See Eye to Eye
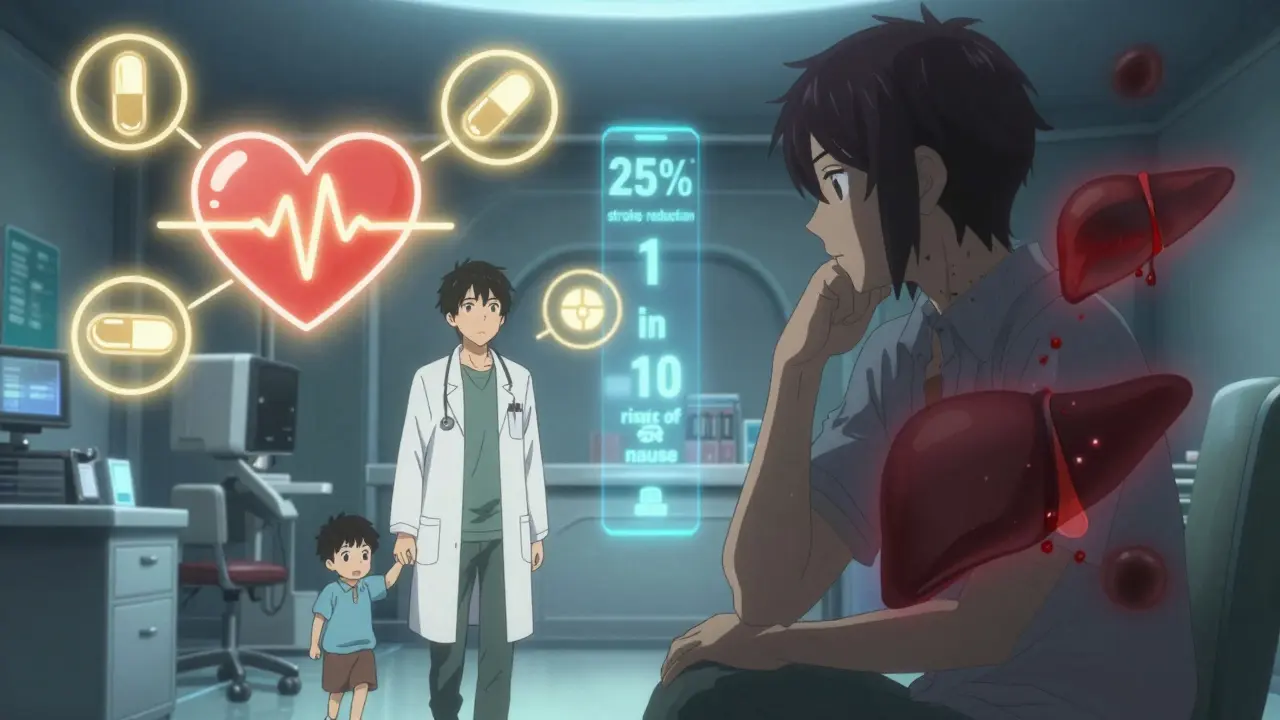
Here’s where things get messy. Patients and doctors don’t weigh risks the same way. A 2023 study from the Michael J. Fox Foundation found that Parkinson’s patients were willing to accept a 20% risk of uncontrollable movements (dyskinesia) for a 30% improvement in mobility. Doctors? They thought patients would only accept 12%. Why the gap? Patients aren’t just thinking about survival-they’re thinking about quality. Can they hold their grandchild’s hand? Walk without help? Eat without choking? Those things matter more than a percentage point on a chart. On the flip side, a patient with high blood pressure might refuse an ACE inhibitor because they heard about a 0.1% chance of angioedema-a rare but scary swelling of the throat. What they don’t realize? That same drug cuts stroke risk by 25%. For someone with high blood pressure, that’s a huge win. But fear doesn’t care about statistics. It cares about stories. That’s why doctors now use visual tools-like charts showing “1 in 10” instead of “10%”-to help patients understand real risk. The FDA has built 47 of these patient decision aids. At Mayo Clinic, they cut medication non-adherence by 22% just by making the numbers clearer.
Why Some Drugs Get Approved-and Others Don’t
Not every drug that works gets approved. The FDA rejected dozens of drugs in 2022-not because they didn’t work, but because the risks were too high for the benefit. Take new cardiovascular drugs. They lower cholesterol or blood pressure, but they also increase bleeding risk. For someone with a history of heart attack? The benefit wins. For someone with no heart disease, just high cholesterol? The bleeding risk isn’t worth it. That’s why the FDA held back on some of these drugs in 2022-they were designed for healthy people, not sick ones. On the other hand, Zolgensma, a gene therapy for spinal muscular atrophy, costs $2.1 million. It can cause serious liver damage. But without it, babies with this condition die or become permanently paralyzed. For a disease that affects 1 in 10,000 births, the price and risk were accepted. Why? Because there was nothing else.The Hidden Cost: Time, Money, and Inequality
Benefit-risk decisions aren’t just clinical-they’re economic. Pharmaceutical companies spend an average of $150 million per drug on post-market safety studies. Complex safety programs can cost half a billion dollars a year. That’s why drugs for rare diseases often come with sky-high prices. The market is small, but the risks are high. And here’s the problem: the data isn’t fair. Clinical trials are still mostly made up of white patients. But minorities make up 40% of the U.S. population. A drug that’s safe for one group might be riskier for another. A 2023 JAMA study found that current risk models often miss this, leading to inaccurate safety predictions for Black, Hispanic, and Indigenous patients. Doctors are starting to notice. More are asking: “Did this drug work in people like my patient?” If the answer is no, they’re hesitant to prescribe it-even if the label says it’s approved.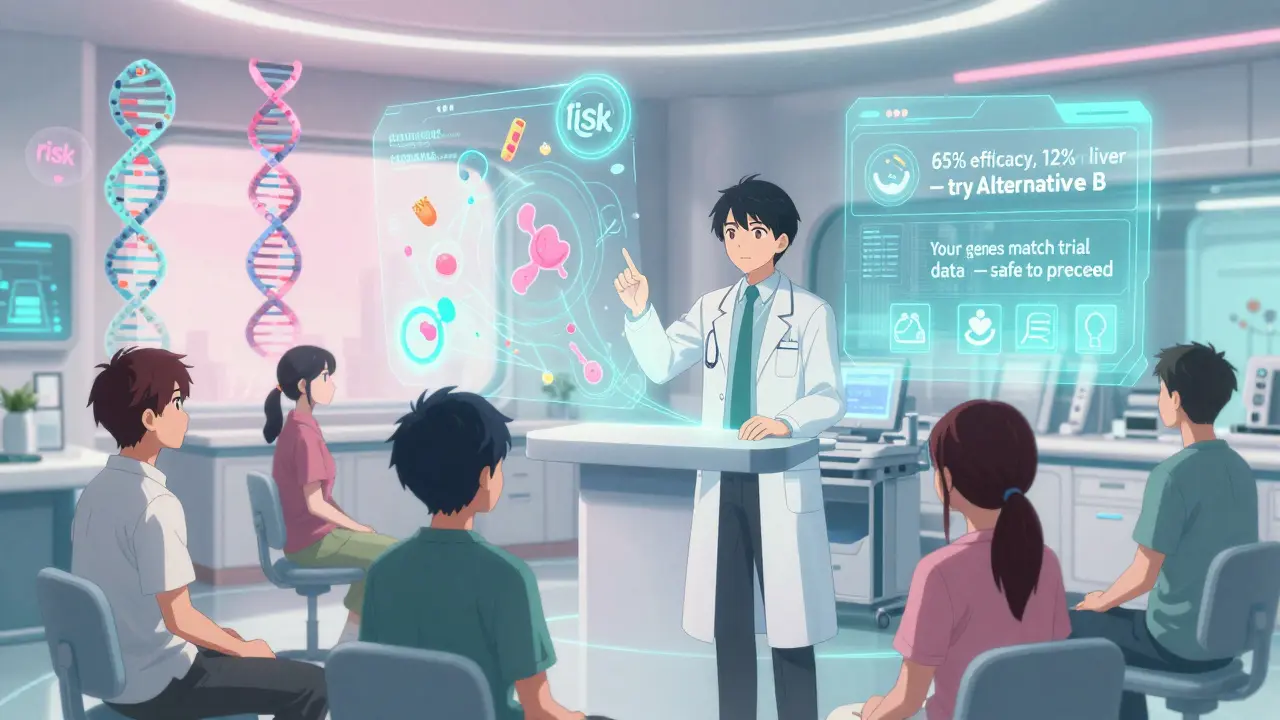
What’s Next? Personalized Risk
The future of medication safety isn’t one-size-fits-all. By 2030, experts predict 70% of benefit-risk assessments will include your DNA, your lifestyle, your other medications, even your gut bacteria. Imagine a tool that says: “Based on your genes, this drug has a 12% chance of causing liver damage-but a 65% chance of working. Here’s a safer alternative.” AI is already helping. Roche’s ARIA platform uses real-world data to spot side effects earlier. The FDA is testing real-world evidence from electronic health records to speed up decisions. And patient voices? They’re now part of the approval process. In rare diseases, 85% of patient preference studies have directly influenced drug labeling.What This Means for You
You don’t need to be a doctor to understand this. When you’re prescribed a new medication, ask:- What’s the main benefit? (e.g., “Reduces stroke risk by 25%”)
- How common are the side effects? (Ask for numbers: “1 in 10” not “some people”)
- Are there safer options? What happens if I don’t take it?
- Does this work for people like me? (Age, gender, race, other conditions)
Medications aren’t magic bullets. They’re tools. And like any tool, they’re only as good as the judgment behind their use. The best doctors don’t just know the science-they know the person.
Why do doctors prescribe drugs with serious side effects?
Doctors prescribe drugs with serious side effects when the condition being treated is life-threatening or severely limiting, and there are no safer alternatives. For example, chemotherapy causes nausea, hair loss, and fatigue-but for many cancers, it’s the only thing that extends life. The decision is based on whether the benefit (like survival or symptom relief) outweighs the harm. It’s not about ignoring risk-it’s about managing it in context.
Are side effects always listed in the patient leaflet?
Yes, all known side effects from clinical trials must be listed in the patient information leaflet. But some rare or long-term effects may not show up until after the drug is widely used. That’s why post-market surveillance is critical. The FDA and drugmakers keep tracking side effects for years after approval. If a new serious risk appears, the label gets updated, and doctors are notified.
Can I refuse a medication because of the side effects?
Absolutely. You have the right to refuse any medication, even if your doctor recommends it. But it’s important to understand the consequences. For example, refusing blood thinners after a stroke might increase your risk of another stroke by 70%. Talk to your doctor about alternatives, or ask for a second opinion. Your choice matters-but so does the information behind it.
Why do some people tolerate side effects better than others?
Genetics, age, liver and kidney function, and other medications all play a role. Some people metabolize drugs faster, others slower. A side effect that’s mild for one person could be dangerous for another. That’s why doctors start with low doses and adjust based on how you respond. Personalized medicine is making this easier-testing your genes before prescribing is becoming more common, especially in cancer and mental health.
How do I know if the benefit is real or just hype?
Look for numbers, not marketing. Ask: “What’s the actual improvement?” For example, “This drug reduces heart attack risk by 10%” sounds good-but if your baseline risk is 2%, you’re going from 2% to 1.8%. That’s a small gain. But if your risk is 20%, a 10% reduction means dropping to 18%. That’s meaningful. Ask your doctor to explain the numbers in terms of real outcomes-not percentages alone.

Doctors don't prescribe drugs because they're perfect-they prescribe them because the alternative is worse. I had a cousin on chemo for stage IV lung cancer; she lost her hair, her appetite, her energy-but she held her granddaughter's wedding. That's the trade-off. No pill is magic. But sometimes, it's the only thing standing between you and goodbye.
Yeah, this is basically how I see it too. I used to think meds were either good or bad. Now I get it-they're tools. Like a hammer. You don't avoid hammers because they can smash your thumb. You use them when you need to build something.
The clinical framework outlined here is methodologically sound, yet it remains insufficient without contextualizing socioeconomic determinants of health. Access to follow-up care, cultural competency in patient education, and pharmaceutical pricing structures significantly modulate the risk-benefit calculus in real-world practice.
I appreciate the emphasis on personalized risk assessment. In India, we often see patients being prescribed Western-developed drugs without considering genetic variations in metabolism-especially in South Asian populations. A drug that's safe for a white patient may cause severe hepatotoxicity in someone with a different CYP450 profile. We need more inclusive trials.
This is so important!! I used to be terrified of every side effect listed in the leaflet-until my doctor showed me a chart: '1 in 10' vs. '10%'. Suddenly it made sense. I started asking for numbers, not vibes. And guess what? I'm still alive, and my blood pressure is stable. Knowledge is power, people!! 💪❤️
They’re hiding the truth. Big Pharma knows these drugs are poison. They just make the side effects sound like ‘mild nausea’ when it’s actually ‘your liver turns to mush in 3 months’. They don’t want you to know. That’s why they silence whistleblowers. Watch the documentaries. They’re not lying to you… they’re lying to EVERYONE. 😡💉
Oh wow, so now we're giving doctors the power to play god with our bodies based on 'risk models'? And you think this is progress? We used to die from infections. Now we die from the cure. The real benefit? Shareholder dividends. The real risk? Your life being reduced to a spreadsheet. 🤡
you’re not alone if you’re scared of meds. i was too. but talking to my doc and asking for the numbers helped so much. you got this. take your time. you don’t have to decide today. ❤️
They say 'benefit outweighs risk' but what if the risk is you become a zombie? I took that blood pressure pill and I couldn't get hard for 6 months. No one told me that. Just said 'dizziness'. Bullshit. They're covering up the real side effects. I'm not the only one. Google 'SSRI sexual dysfunction'. They don't want you to know.
Love this breakdown! I always say: if your doctor doesn’t explain the 'why' behind the med, find a new one. My aunt refused her statin because she read a blog about liver damage. Turns out her risk of heart attack was 3x higher. We sat down with a chart and she cried. Then she took it. Now she’s hiking at 78. 😊
Ugh, I just had to sit through 20 minutes of my doctor explaining 'risk-benefit' like I'm a child. Like I don't know my own body?! I'm not a statistic. I'm a PERSON. And my anxiety about side effects? That's real too. Why don't they ever talk about that? 🤍
Every pill is a conversation between biology and choice. We forget that medicine isn't just science-it's a mirror of our fears, our hopes, our cultural stories. The real question isn't 'does it work?' but 'what are you willing to lose to keep living?' 🙏
The inclusion of real-world data and patient preference studies in drug approval is a significant advancement. It acknowledges that medical decisions are not purely clinical but deeply human. This shift toward participatory medicine must be expanded globally, especially in low-resource settings where access to information remains limited.
omg yes!! i had a doc who just handed me a script and said 'take this'. i said no. asked for alternatives. they were annoyed. but i found a different med that didn't make me feel like a zombie. you deserve to be heard. don't let anyone rush you. 💪