How Brain Tumors Trigger Central Cranial Diabetes Insipidus - Causes, Diagnosis, and Treatment

Central DI Fluid Management Calculator
Fluid Balance Calculator
Track your fluid intake and output to maintain safe hydration levels. This tool helps calculate your daily fluid balance based on urine output and serum sodium levels.
Fluid Balance Assessment
This tool provides general guidance only. Always consult your healthcare provider for personalized medical advice.
Recommendations
When a brain tumor messes with the tiny pathways that control water balance, the result can be a baffling condition called Central Cranial Diabetes Insipidus - a disorder where the body can’t retain water because it lacks the hormone vasopressin. Understanding why tumors cause this problem, how doctors spot it, and what treatment looks like can turn a scary diagnosis into a manageable plan.
Key Takeaways
- Central DI occurs when the posterior pituitary or its stalk is damaged, often by a tumor.
- Craniopharyngioma, meningioma, and pituitary adenoma are the most frequent culprits.
- Diagnosis relies on water‑deprivation tests, serum sodium, and high‑resolution MRI of the sellar region.
- Desmopressin is the first‑line therapy; surgery or radiotherapy addresses the underlying tumor.
- Long‑term follow‑up is essential because tumor recurrence can reignite DI symptoms.
What Is Central Cranial Diabetes Insipidus?
Central Cranial Diabetes Insipidus (often shortened to central DI) is a deficiency of antidiuretic hormone (ADH), also called vasopressin, which is produced in the hypothalamus and stored in the posterior pituitary. Without enough ADH, the kidneys can’t re‑absorb water, leading to excessive urine output (polyuria) and extreme thirst (polydipsia). Unlike the more common type1 diabetes, DI does not involve blood‑sugar problems.
Typical lab findings include low urine osmolality, high serum sodium (hypernatremia), and a marked rise in urine volume-often over 3liters per day. Left unchecked, dehydration can become life‑threatening, especially in children or the elderly.
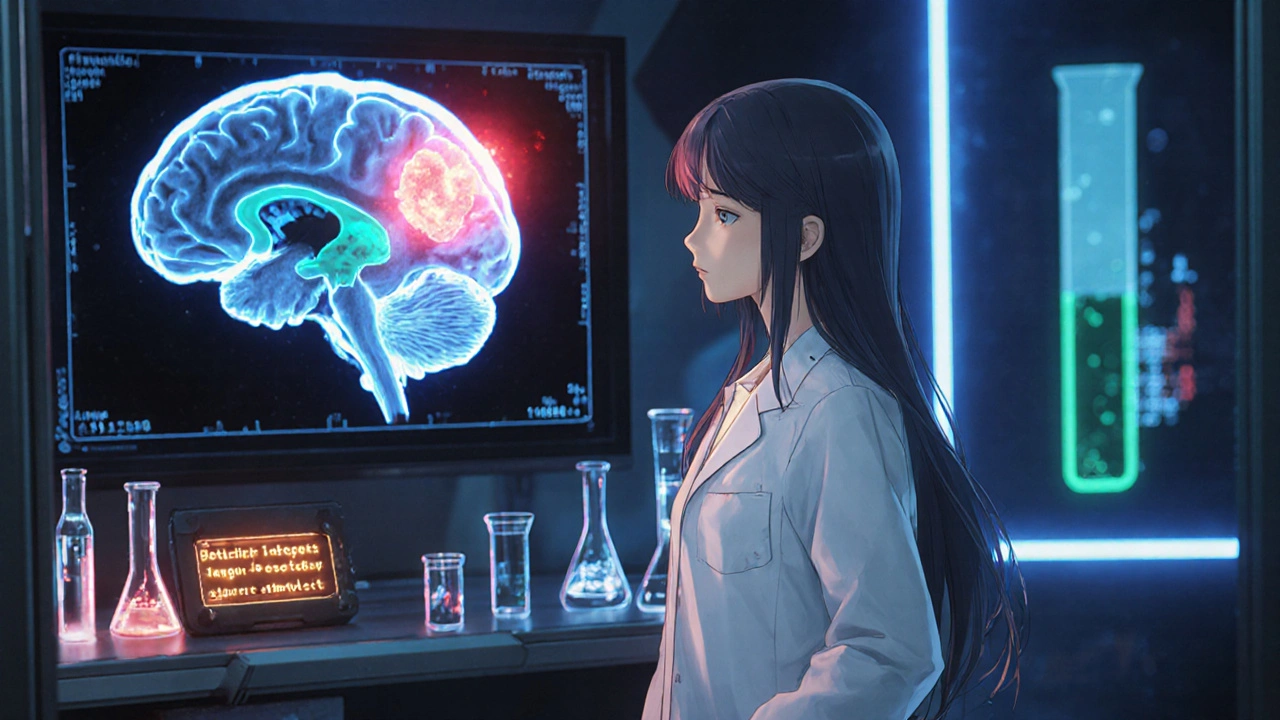
How Brain Tumors Lead to Central DI
Brain tumors can disrupt the ADH pathway in three main ways:
- Compression of the pituitary stalk: The thin infundibulum carries ADH from the hypothalamus to the posterior pituitary. A mass in the suprasellar region squeezes the stalk, cutting off hormone transport.
- Direct invasion of the posterior pituitary: Some tumors grow into the gland itself, destroying the cells that release ADH.
- Post‑surgical or radiation injury: Treatment of a tumor can inadvertently damage the delicate neuro‑vascular structures that regulate ADH.
Because the hypothalamic‑pituitary axis sits in a tight space, even a small lesion can have outsized effects on water balance.
Common Tumor Types Linked to Central DI
Not all brain tumors cause DI, but several have a strong association:
| Tumor | Typical Location | How It Triggers DI | Incidence in DI Patients |
|---|---|---|---|
| Craniopharyngioma | Suprasellar region | Stalk compression, pituitary invasion | ≈30% |
| Meningioma | Parasellar or tuberculum sellae | Mass effect on infundibulum | ≈20% |
| Pituitary adenoma (macro) | Pituitary fossa | Posterior gland involvement after surgery | ≈15% |
| Glioma (low‑grade) | Hypothalamic region | Direct hypothalamic damage | ≈10% |
| Langerhans cell histiocytosis | Skull base | Inflammatory infiltration of stalk | ≈5% |
Craniopharyngioma tops the list because it almost always sits right above the pituitary stalk. Meningiomas are the second most common, especially those that grow near the cavernous sinus.

Diagnosis: Imaging & Laboratory Tests
Diagnosing central DI in the context of a brain tumor follows a two‑pronged approach: hormone testing and imaging.
- Water‑deprivation test: Patients are monitored while fluid intake is restricted. In central DI, urine remains dilute despite rising serum osmolality.
- Desmopressin (DDAVP) challenge: After giving a small dose of synthetic ADH, urine osmolality should rise sharply if the kidneys are functional.
- Serum electrolytes: Hypernatremia (>145mmol/L) supports the diagnosis.
- Magnetic Resonance Imaging (MRI): A high‑resolution scan of the sellar and suprasellar regions visualizes the tumor, pituitary stalk thickness, and posterior pituitary bright spot. Loss of the bright spot often correlates with central DI.
When MRI reveals a lesion, a multidisciplinary team-neuroradiologist, endocrinologist, and neurosurgeon-decides the next steps.
Treatment Options: Managing DI and the Tumor
Therapy splits into two goals: replace missing ADH and treat the tumor.
1. Desmopressin Replacement
Desmopressin, a synthetic analog of vasopressin, is given as a nasal spray, oral tablet, or sublingual melt. Dosing starts low (0.1mg nasal) and is titrated to keep urine output under 2L/day and serum sodium in the normal range. Over‑replacement can cause hyponatremia, so patients are taught to monitor weight and fluid intake.
2. Tumor‑Directed Therapy
- Surgical resection: For accessible tumors like craniopharyngioma, endoscopic trans‑sphenoidal surgery removes the mass and often relieves stalk compression. However, surgery can also damage the posterior pituitary, sometimes worsening DI.
- Radiation therapy: Fractionated stereotactic radiotherapy targets residual tumor while sparing surrounding tissue. It’s useful when complete resection isn’t possible.
- Chemotherapy/Targeted agents: Rarely used for DI‑causing tumors, but certain gliomas respond to temozolomide or newer molecular inhibitors.
In many cases, treating the tumor improves DI symptoms, but a proportion of patients remain dependent on desmopressin permanently.
3. Managing Complications
Patients on desmopressin need regular checks for:
- Hyponatremia - especially after surgery or during illness.
- Fluid overload - avoid excessive water intake when ADH levels rise suddenly.
- Electrolyte shifts - monitor potassium and calcium if diuretics are used for other reasons.

Prognosis and Follow‑up Care
Long‑term outlook depends on tumor type and treatment success. For benign tumors like craniopharyngioma, five‑year survival exceeds 90%, but endocrine deficits often persist. Malignant gliomas carry a poorer overall prognosis, and DI may be just one of many neurological issues.
Follow‑up typically includes:
- Quarterly endocrine panels for the first year, then semi‑annual if stable.
- Annual MRI to watch for tumor recurrence.
- Patient education on signs of over‑ or under‑ replacement (headache, nausea, rapid weight change).
Living with central DI after a brain tumor is a team effort-endocrinologists adjust medication, neurosurgeons monitor imaging, and primary care keeps an eye on overall health.
Frequently Asked Questions
Can central DI develop years after tumor treatment?
Yes. Radiation‑induced fibrosis or delayed scar tissue can compress the pituitary stalk many months-or even years-after the initial therapy, leading to new‑onset DI.
Is desmopressin safe during pregnancy?
Desmopressin is classified as CategoryB, meaning animal studies show no risk and there are no well‑controlled human studies. Most endocrinologists continue it if the benefits outweigh potential risks.
What lifestyle changes help control DI symptoms?
Drink fluids regularly but avoid binge‑drinking large volumes at once. Keep a water‑intake log, weigh yourself daily, and carry a medical alert bracelet indicating desmopressin use.
Can other brain conditions cause central DI?
Besides tumors, traumatic brain injury, sarcoidosis, and infections like meningitis can disrupt the hypothalamic‑pituitary axis and trigger DI.
How is central DI different from nephrogenic DI?
Central DI stems from a lack of ADH production, while nephrogenic DI occurs when kidneys ignore ADH due to receptor defects or drug side‑effects. Desmopressin works well for central but has limited effect on nephrogenic forms.
Understanding the tie between brain tumors and central cranial diabetes insipidus equips patients and caregivers to catch symptoms early, pursue the right tests, and stick to a treatment plan that balances hormone replacement with tumor control.


Our heroes in medicine must rally!!! The link between suprasellar tumours and the loss of that precious hormone-vasopressin-demands immediate national attention!!! Every missed diagnosis is a betrayal of our citizens!!! We cannot tolerate delays; the water‑deprivation test must be ordered at once!!!
Certainly, the desmopressin dosage should be titrated carefully; the patient’s serum sodium must remain within normal limits, and any deviation should be corrected promptly. Additionally, the endocrine team should involve the neurosurgeon early. Please note that the water‑deprivation test remains gold‑standard. Also, postoperative monitoring definatly includes MRI.
The hypothalamic‑pituitary circuitry is a high‑stakes conduit for antidiuretic signaling, and any neoplastic intrusion can trigger a cascade of osmotic dysregulation 😱. Compression of the infundibular stalk impairs vasopressin trafficking, resulting in polyuria that overwhelms renal concentrating capacity 📊. Moreover, radiation‑induced fibro‑blasts may remodel the stalk long after the primary resection, creating a delayed‑onset DI scenario 🧬. Clinicians must therefore schedule serial MRI assessments and maintain a vigilant eye on serum osmolality trends to preempt catastrophic dehydration 🔍. In practice, a multidisciplinary tumor board should evaluate each case for both oncologic control and endocrine preservation 👩⚕️👨⚕️.
The posterior pituitary injury directly reduces ADH output.
ADH deficit leads to polyuria and hypernatremia; clinicians must monitor osmolality as a key biomarker.
Let me be clear-ignoring the endocrine fallout of a craniopharyngioma is a selfish act against our great nation’s health!!! The bright‑spot loss on MRI is not just a radiologic curiosity, it’s a red flag that screams for immediate hormonal rescue!!! You cant just "watch and wait" when the patient is spilling liters of urine daily-action is mandatory!!!
The phenomenon of central diabetes insipidus, when caused by a brain tumor, illustrates the delicate interdependence of structure and function within the hypothalamic‑pituitary axis. A tumor compressing the infundibulum acts as a physical barrier, severing the neurovascular conduit that transports vasopressin from the supraoptic nuclei to the posterior lobe. This interruption curtails the secretion of antidiuretic hormone, leading to an inability of the kidneys to reabsorb water and consequently producing polyuria. The resultant hypernatremia further stresses homeostatic mechanisms, demanding swift clinical intervention. Diagnosis hinges on the water‑deprivation test, which distinguishes central from nephrogenic forms by assessing urinary concentration in response to rising serum osmolality. A positive response to desmopressin confirms central etiology, as the renal collecting ducts remain responsive to exogenous ADH. Imaging, particularly high‑resolution MRI, visualizes the lesion, identifies stalk thickening, and may reveal loss of the posterior pituitary bright spot, a surrogate marker of ADH storage depletion. Surgical resection, when feasible, can alleviate mass effect, yet the procedure itself carries a risk of further damaging the delicate hypothalamic‑pituitary interface. Radiotherapy offers an alternative for residual disease but may induce delayed fibrosis, perpetuating DI. Long‑term management frequently requires lifelong desmopressin therapy, calibrated to maintain euvolemia without precipitating hyponatremia. Regular monitoring of serum sodium, urine output, and body weight is essential to adjust dosing. Multidisciplinary collaboration among endocrinologists, neurosurgeons, and radiologists optimizes outcomes and minimizes complications. Patient education on fluid intake patterns and symptom recognition empowers individuals to self‑manage their condition. Future research into neuroprotective agents may one day preserve vasopressin pathways during tumor treatment. Until such advances materialize, vigilant assessment and personalized hormone replacement remain the cornerstone of care.